Program Overview
Addressing a crisis of the magnitude and urgency of the COVID-19 pandemic transcends what any single entity can accomplish alone. It calls for the rapid and efficient mobilization and coordination of our nation's clinical trial infrastructure. The NHLBI Collaborating Network of Networks for Evaluating COVID-19 and Therapeutic Strategies (CONNECTS) was formed with the aim of helping prevent infection, slow or halt disease progression, and speed recovery.
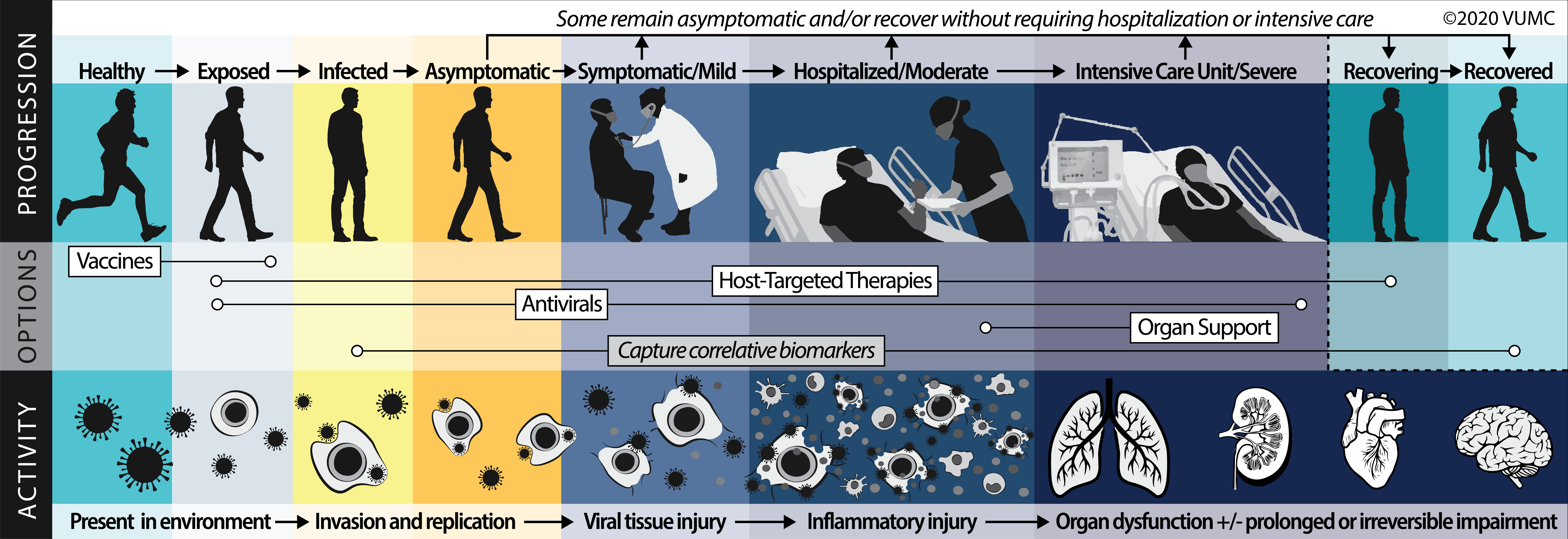
The CONNECTS model promotes seamless collaboration by fully integrating major NHLBI clinical trial networks under one organizational umbrella. An adaptive platform, master-protocol driven approach targets critical points along the clinical spectrum, from exposure to development of mild symptoms, to development of symptoms requiring hospitalization, and extending through convalescence and recovery. CONNECTS rigorously tests trials with the following COVID-19 host-directed therapeutics:
- Passive Immunity
- Anticoagulation
- Immunomodulation
- Host-Tissue Directed
The adaptive platform setting will allow for the rapid removal of ineffective therapies and the fluid introduction of new, promising treatments. By partnering with NHLBI Biodata Catalyst, a shared virtual space where scientists can access and work with the digital objects of biomedical research, CONNECTS ensures efficiencies in standardization and sharing of both resources and data. CONNECTS is also working in close collaboration with the FDA, ACTIV, BARDA, and Operation Warp Speed to significantly contribute to the evidence base for clinical practice and the development of safe and effective interventions and implementation strategies to conquer COVID-19.
Governance
NHLBI CONNECTS Governance and Oversight
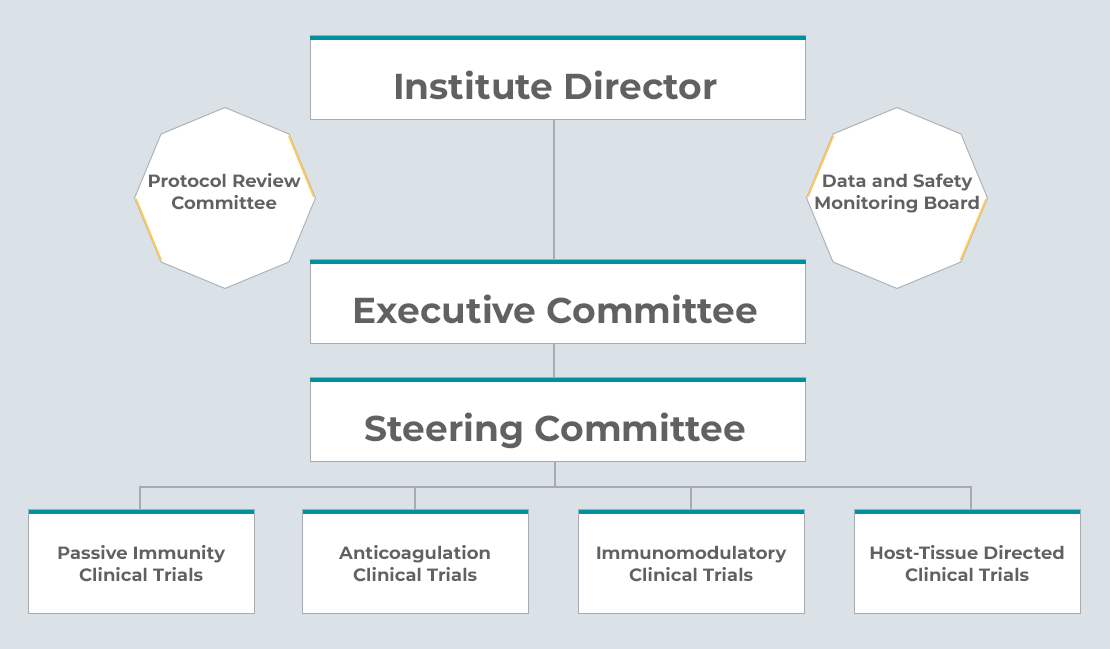
Executive Committee (EC)
The CONNECTS EC is responsible for ensuring the program effectively meets its objectives and mission, proposing solutions to challenges that arise, and providing leadership for final programmatic decisions.
Steering Committee (SC)
The CONNECTS SC is responsible for the design, implementation, and analysis of CONNECTS study protocols, as well as development of standards for sharing of data, biospecimens, and other research products.
