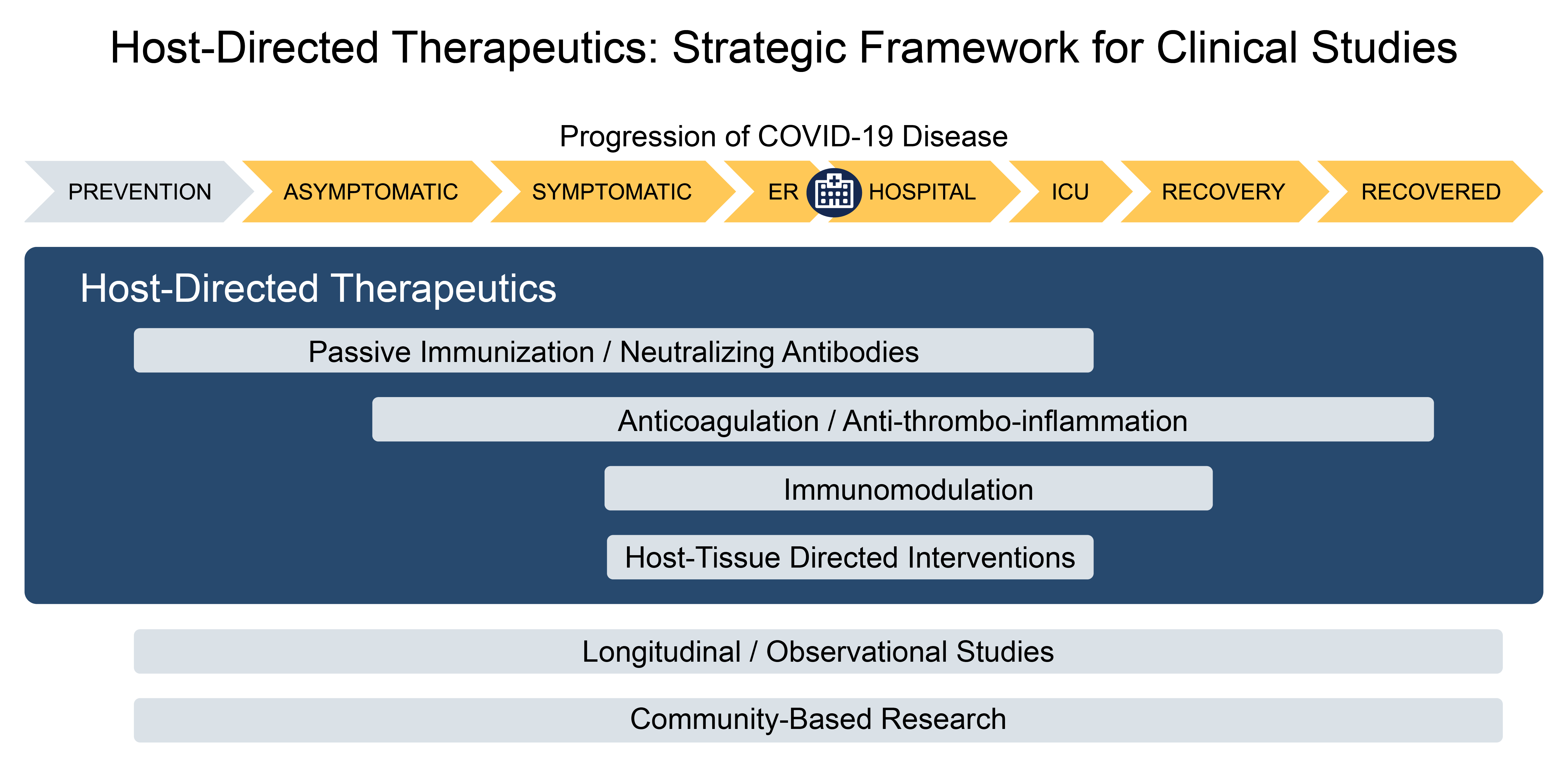
Therapeutic Framework
The CONNECTS framework includes four therapeutic domains within which clinical trials will be conducted: passive immunity, anticoagulation, immunomodulation, and host-tissue targeted therapies.
Passive Immunity: Development of neutralizing antibodies against SARS-CoV-2 may occur after infection. Passive immunization with convalescent plasma (from individuals who have recovered from COVID-19) and monoclonal antibodies (mAbs) that neutralize SARS-CoV-2 represent promising passive immunity therapies.
Anticoagulation: Hypercoagulability in COVID-19 has been shown to play a significant role in overall outcomes, and incidence of thrombotic disease has been reported in up to a third of COVID-19 patients. Therapies in this category include warfarin, heparin and derivative substances, inhibitors of factor Xa, directly acting oral anticoagulants (DOACs), and antithrombin protein therapeutics.
Immunomodulatory: Patients with severe or later-stage COVID-19 can develop a syndrome of dysregulated and systemic immune overactivation that worsens acute respiratory distress syndrome and can lead to multisystem organ failure. Approved drugs that modulate targets within the inflammatory system include interferon beta, adalimumab, colchicine, tocilizumab, anakinra, and nivolumab.
Host-Tissue Targeted Therapeutics: The broadest of the COVID-19 therapeutic categories includes therapies that target pathways involved in viral damage to host tissue. Examples include modulators of the Renin-Angiotensin-Aldosterone System (RAAS), Transmembrane Serine Protease 2 inhibitors such as camostat, neutrophil elastase inhibitors, and others.